In Silico Molecular Interaction of Phenyl Alkyl Caffeine Derivatives as Monoamine Oxidase B Inhibitors Research Article
Main Article Content
Abstract
Background: Neurodegenerative disorders often involve increased monoamine oxidase B (MAO-B) activity, which leads to excessive reactive oxygen species (ROS) and cell damage. Neuroprotection through ROS inhibition can be achieved with MAO-B inhibitors, helping to reduce motor symptoms in Parkinson’s disease, amyloid plaques in Alzheimer’s disease, and impairment in non-motor issues such as mood, cognition, sleep, and fatigue.
Methods: This study investigated the interaction and inhibitory potential of C8-substituted phenyl alkyl caffeine derivatives against monoamine oxidase B (MAO-B) using molecular docking. Docking was performed through the Virtual Screening interface of PyRx, integrated with the AutoDock Vina engine, to evaluate binding affinity and active-site interactions. Additionally, in-silico absorption, distribution, metabolism, and excretion (ADME) profiling and drug-likeness assessments were conducted to determine their suitability as potential reversible MAO-B inhibitors.
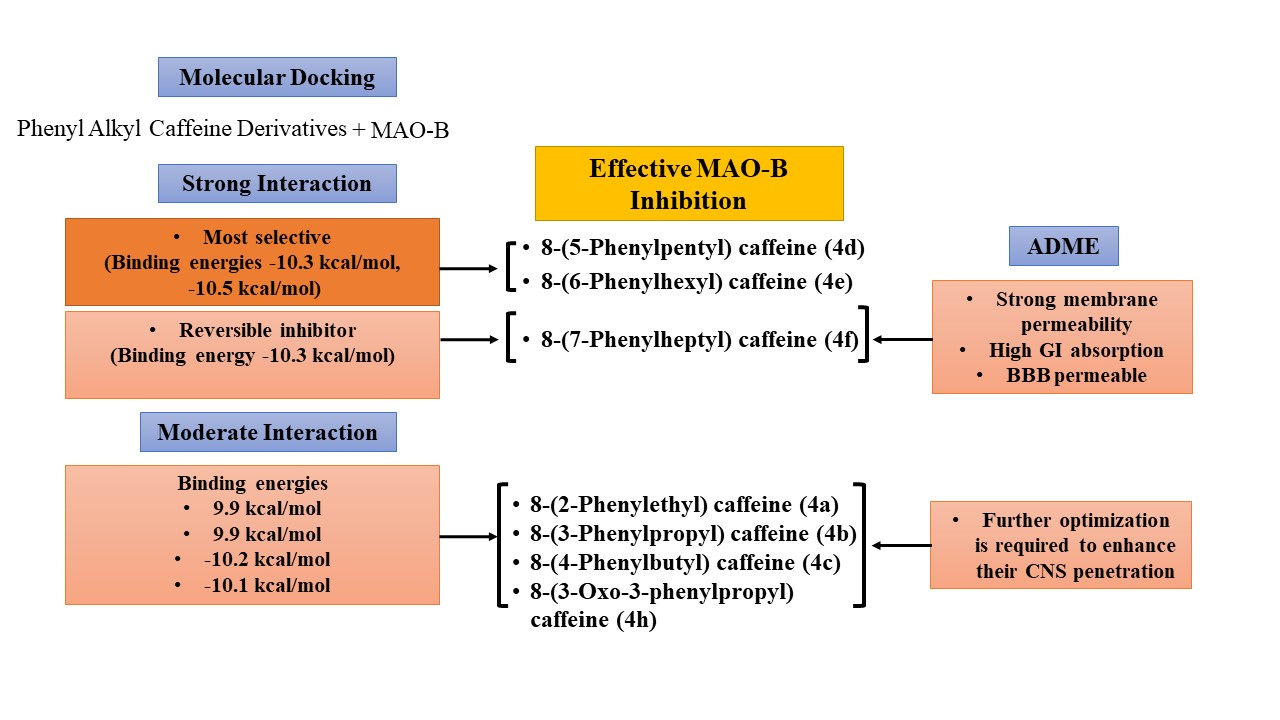
Results: Caffeine analogs with shorter substituents (4a, 4b) showed moderate interactions with the amino acids in the substrate cavity of MAO-B, whereas derivatives with longer substituents, 8-(5-Phenylpentyl) caffeine (4d), (4e), and (4f), exhibited robust binding affinities, -10.3, -10.5, and -10.3 kcal/mol, respectively. Among these compounds, 4d emerged as the most selective inhibitor, forming strong, stable conventional hydrogen bonds. 4e and 4f also exhibited substantial binding energies. 4i displayed strong interactions with slightly lower efficacy. ADME analysis revealed 4f with high gastrointestinal (GI) absorption and blood-brain barrier (BBB) permeability, suggesting potential for CNS-targeted applications.
Conclusion: This study demonstrates that 8-(5-Phenylpentyl) caffeine (4d) and 8-(5-Phenylpentyl) caffeine (4e) are highly selective and potent inhibitors of MAO-B. Among all ligands, 8-(7-Phenylheptyl) caffeine (4f) stood out as a reversible inhibitor, having high GI and BBB permeability. These findings highlight the importance of designing selective and multifunctional MAO-B inhibitors for effective neuroprotective therapy.
Article Details
Section

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
References
1. Shahid R, Begum S. A New Insight in Cellular and Molecular Signaling Regulation for Neural Differentiation Program. Molecular neurobiology. 2025;20:1-22.
2. Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nature reviews neuroscience. 2006;7(4):295-309.
3. Nebbioso M, Pascarella A, Cavallotti C, Pescosolido N. Monoamine oxidase enzymes and oxidative stress in the rat optic nerve: age-related changes. International journal of experimental pathology. 2012;93(6):401–5.
4. Huang M, Xie SS, Jiang N, Lan JS, Kong LY, Wang XB, Wang. Multifunctional coumarin derivatives: monoamine oxidase B (MAO-B) inhibition, anti-β-amyloid (Aβ) aggregation and metal chelation properties against Alzheimer’s disease. Bioorganic and medicinal chemistry letters. 2015;25(3):508–13.
5. Noda S, Sato S, Fukuda T, Tada N, Uchiyama Y, Tanaka K, Hattori N. Loss of Parkin contributes to mitochondrial turnover and dopaminergic neuronal loss in aged mice. Neurobiology of disease. 2020;136:104717.
6. Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. British journal of pharmacology. 2006;147(Suppl 1):S287–96.
7. Tetrud JW, Koller WC. A novel formulation of selegiline for the treatment of Parkinson’s disease. Neurology. 2004;63(7 Suppl 2):S2–6.
8. Carradori S, D’Ascenzio M, Chimenti P, Secci D, Bolasco A. Selective MAO-B inhibitors: a lesson from natural products. Molecular diversity. 2014;18(1):219–43.
9. Chandran N, Lee J, Prabhakaran P, Kumar S, Sudevan ST, Parambi DG, Alsahli TG, Pant M, Kim H, Mathew B. New class of thio/semicarbazide-based benzyloxy derivatives as selective class of monoamine oxidase-B inhibitors. Scientific reports. 2024;14(1):31292.
10. Parkinson Study Group. Effect of deprenyl on the progression of disability in early Parkinson's disease. New England journal of medicine. 1989;321(21):1364–71.
11. Poewe W, Seppi K, Fitzer-Attas CJ, Wenning GK, Gilman S, Low PA, Giladi N, Barone P, Sampaio C, Eyal E, Rascol O. Efficacy of rasagiline in patients with the parkinsonian variant of multiple system atrophy: a randomised, placebo-controlled trial. The lancet neurology. 2015;14(2):145-52.
12. Khanam S, Subitsha AJ, Sabu S. Plants as a promising source for the treatment of Parkinson disease: a systematic review. International journal of comprehensive and advanced pharmacology. 2021;5(3):158–66.
13. Tipton KF, Boyce S, O'Sullivan J, Davey GP, Healy J. Monoamine oxidases: certainties and uncertainties. Current medicinal chemistry. 2004;11(15):1965–82.
14. Fowler JS, Volkow ND, Logan J, Wang GJ, MacGregor RR, Schlyer D. Slow recovery of human brain MAO B after L-deprenyl (Selegiline) withdrawal. Synapse. 1994;18(1):86–93.
15. Alborghetti M, Bianchini E, De Carolis L, Galli S, Pontieri FE, Rinaldi D. Type-B monoamine oxidase inhibitors in neurological diseases: clinical applications based on preclinical findings. Neural regeneration research. 2024;19(1):16-21.
16. Smellie FW, Davis CW, Daly JW, Wells JN. Alkylxanthines: inhibition of adenosine-elicited accumulation of cyclic AMP in brain slices and of brain phosphodiesterase activity. Life sciences. 1979;24(26):2475–82.
17. Boulenger JP, Patel J, Marangos PJ. Effects of caffeine and theophylline on adenosine and benzodiazepine receptors in human brain. Neuroscience letters. 1982;30(3):161–6.
18. Vlok N, Malan SF, Castagnoli N Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase B by analogues of the adenosine A2A receptor antagonist (E)-8-(3-chlorostyryl) caffeine (CSC). Bioorganic and medicinal chemistry. 2006;14(11):3512–21.
19. Strydom B, Malan SF, Castagnoli N Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase by 8-benzyloxycaffeine analogues. Bioorganic and medicinal chemistry. 2010;18(3):1018–28.
20. Pretorius J, Malan SF, Castagnoli N Jr, Bergh JJ, Petzer JP. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A2A receptor by (E, E)-8-(4-phenylbutadien-1-yl) caffeine analogues. Bioorganic and medicinal chemistry. 2008;16(19):8676–84.
21. Mateev E, Kondeva-Burdina M, Georgieva M, Zlatkov A. Repurposing of FDA-approved drugs as dual-acting MAO-B and AChE inhibitors against Alzheimer's disease: an in silico and in vitro study. Journal of molecular graphics and modelling. 2023;122:108471.
22. Alagöz MA, Oh JM, Zenni YN, Özdemir Z, Abdelgawad MA. Development of a novel class of pyridazinone derivatives as selective MAO-B inhibitors. Molecules. 2022;27(11):3801.
23. Venkidath A, Oh JM, Dev S, Amin E, Rasheed SP, et al. Selected class of enamides bearing nitro functionality as dual-acting with highly selective monoamine oxidase-B and BACE1 inhibitors. Molecules. 2021;26(19):6004.
24. Binda C, Newton-Vinson P, Hubálek F, Edmondson DE, Mattevi A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nature structural biology. 2002;9(1):22–6.
25. Binda C, Wang J, Pisani L, Caccia C, Carotti A, Salvati P. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: safinamide and coumarin analogs. Journal of medicinal chemistry. 2007;50(24):5848–52.
26. Mostert S, Petzer A, Petzer JP. Indanones as high-potency reversible inhibitors of monoamine oxidase. Chem Med Chem. 2015;10(5):862–73.
27. Edmondson DE, Binda C, Mattevi A. Structural insights into the mechanism of amine oxidation by monoamine oxidases A and B. Archives of biochemistry and biophysics. 2007;464(2):269–76.
28. Fonseca A, Reis J, Silva T, Matos MJ, Bagetta D, Ortuso F. Coumarin versus chromone monoamine oxidase B inhibitors. Journal of medicinal chemistry. 2017;60(18):7206–12.
29. Mateev E, Georgieva M, Mateeva A, Zlatkov A, Ahmad S, Raza K, Azevedo V, Barh D. Structure-based design of novel MAO-B inhibitors: a review. Molecules. 2023;28(12):4814.
30. Shahwan M, Prasad P, Yadav DK, Altwaijry N, Khan MS, Shamsi A. Identification of high-affinity Monoamine oxidase B inhibitors for depression and Parkinson’s disease treatment: bioinformatic approach of drug repurposing. Frontiers in pharmacology. 2024;15:1422080.
31. Binda C, Wang J, Pisani L, Caccia C, Carotti A, Salvati P, Edmondson DE, Mattevi A. Structures of human monoamine oxidase B complexes with selective reversible inhibitors. Biochemistry. 2011;50(23):5846–5852.
32. Yau MQ, Loo JS. Consensus scoring evaluated using the GPCR-Bench dataset: reconsidering the role of MM/GBSA. Journal of computer-aided molecular design. 2022; 36(5):427–41.
33. Sahakyan H. Improving virtual screening results with MM/GBSA and MM/PBSA rescoring. Journal of computer-aided molecular design. 2021;35(6):731–6.
34. BIOVIA, Dassault Systèmes. Discovery Studio modeling environment, release 2021. San Diego: Dassault systèmes; 2021.
35. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness, and medicinal chemistry friendliness of small molecules. Scientific reports. 2017;7:42717.
36. O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: an open chemical toolbox. Journal of cheminformatics. 2011;3:33.
37. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 2001;46(1–3):3–26.
38. Ghose AK, Viswanadhan VN, Wendoloski JJ. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. Journal of combinatorial chemistry. 1999;1(1):55–68.
39. Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. Molecular properties that influence the oral bioavailability of drug candidates. Journal of medicinal chemistry. 2002;45(12):2615–23.
40. Egan WJ, Merz KM, Baldwin JJ. Prediction of drug absorption using multivariate statistics. Journal of medicinal chemistry. 2000;43(15):3867–77.
41. Muegge I, Heald SL, Brittelli D. Simple selection criteria for drug-like chemical matter. Journal of medicinal chemistry. 2001; 44(12):1841–6.
42. Delaney JS. ESOL: estimating aqueous solubility directly from molecular structure. Journal of Chemical information and computer sciences. 2004;44(3):1000–5.
43. Ali J, Camilleri P, Brown MB, Hutt AJ, Kirton SB. Revisiting the general solubility equation: in silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. Journal of chemical information and modeling. 2012;52(2):420–8.
44. Van den Berg D, Zoellner KR, Ogunrombi MO, Malan SF, Terre’Blanche G, Castagnoli N Jr. Inhibition of monoamine oxidase B by selected benzimidazole and caffeine analogues. Bioorganic and medicinal chemistry. 2007;15(11):3692–702.
45. Petzer A, Grobler P, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase by selected phenyl alkyl caffeine analogues. Journal of pharmacy and pharmacology. 2014;66(5):677–87.
46. Rauhamäki S, Postila PA, Niinivehmas S, Kortet S, Schildt E, Pasanen M. Structure–activity relationship analysis of 3-phenylcoumarin-based monoamine oxidase B inhibitors. Frontiers in chemistry. 2018; 6:41.
47. Strydom B, Malan SF, Castagnoli N Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase by 8-benzyloxycaffeine analogues. Bioorganic and medicinal chemistry. 2010;18(3):1018–1028.
48. Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-Å resolution: the control of opening the entry for substrates/inhibitors. Proceedings of the national academy of sciences of the United States of America. 2008;105(15):5739–44.
49. Hu Q, Feng M, Lai L, Pei J. Prediction of drug-likeness using deep autoencoder neural networks. Frontiers in genetics. 2018; 9:511.
50. Arnott JA, Planey SL. The influence of lipophilicity in drug discovery and design. Expert opinion on drug discovery. 2012;7(9):863–75.
51. Waring MJ. Lipophilicity in drug discovery. Expert opinion on drug discovery. 2010; 5(3):235–48.
52. Petzer, JP, Castagnoli KP, Steyn S, Bergh JJ, Castagnoli, N. Inhibition of monoamine oxidase B by 8-benzyl caffeine analogues. Bioorganic and medicinal chemistry letters. 2013; 23(2):520–523.
53. Ramsay, R. R., & Albreht, A. Biochemical and structural characterization of MAO inhibitors for Parkinson’s disease therapy. Frontiers in pharmacology. 2017; 8: 436.
54. Hubálek F, Binda C, Khalil A, Li M, Mattevi A, Castagnoli N, Edmondson DE. Demonstration of isoleucine 199 as a structural determinant for the selective inhibition of human monoamine oxidase B by specific reversible inhibitors. Journal of biological chemistry. 2005;280(17):15761–6.
55. Whelan R, Hargaden GC, Knox AJ. Modulating the blood–brain barrier: a comprehensive review. Pharmaceutics. 2021;13(11):1980.
56. Kadry H, Noorani B, Cucullo L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and barriers of CNS. 2020;17(1):69.
57. Abuhelwa AY, Williams DB, Upton RN, Foster DJ. Food, gastrointestinal pH, and models of oral drug absorption. European journal of pharmaceutics and biopharmaceutics. 2017;112:234-48.